Madorra Home Ultrasound System
Improving Quality of Life After Menopause
“As an emerging medical device company, our choice of partners is key to our success. In addition to Product Creation Studio’s medical device experience, they were a good fit because they shared our enthusiasm about the product’s potential and had a real desire to see it reach key clinical milestones.”
About Madorra
Madorra’s goal is to empower people to live fuller, healthier lives. They are changing the paradigm for treating vaginal dryness by providing a nonhormonal and noninvasive home ultrasound solution to a problem that has previously only been served by pharmaceuticals. Madorra is focused on giving post-menopausal people and breast cancer survivors the power to choose how they approach their vaginal health.
The FDA has granted Madorra “breakthrough device designation” or the company’s non-invasive, home ultrasound device for a subset of women experiencing moderate to severe vulvovaginal atrophy (VVA), a component of genitourinary syndrome of menopause (GSM).
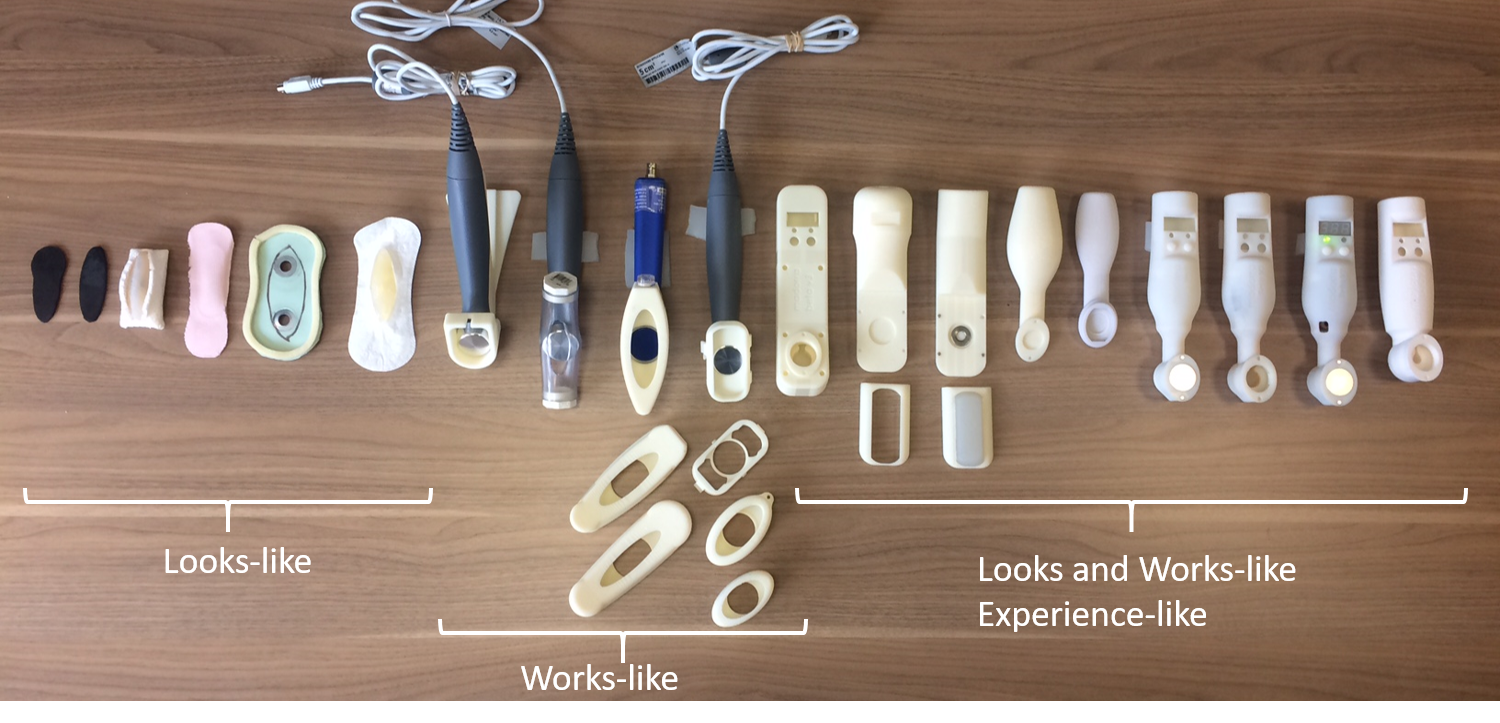
Product Challenge
To support their international clinical study with intended users, Madorra needed to advance their proof-of-concept design into a full-featured home ultrasound device developed under design controls and compliant with industry standards such as IEC 62304:2006 for software and IEC 60601-1-2 EMC for medical electrical equipment. Their upcoming study was a critical strategic milestone, and Madorra recognized the need to level up their design fidelity to support it properly.
Why Madorra Chose Product Creation Studio as Their Development Partner
Product Creation Studio’s experience with hand-held medical devices and therapeutic ultrasound was attractive to the Madorra team. In addition, Product Creation Studio’s quality management system (QMS) that complies with ISO 13485:2016 was perfect for an emerging company like Madorra to take advantage of while they built their own QMS. Flexibility with resource commitments in the face of an evolving scope allowed Madorra to react strategically to the inevitable unknowns of home ultrasound device development.
Product Creation Studio’s Role
Product Creation Studio was asked to bring an early version of the embedded software into compliance with the international standard for medical device software, IEC 62304:2006. This involved leveraging the Product Creation Studio’s software development process to capture a formal software development plan, detailed technical requirements, software architecture, and a full suite of formal verification protocols.
Madorra’s prototype firmware required refactoring and an algorithm update to provide reliable delivery of ultrasound therapy.
Madorra’s commitment to development of a safe and effective device led them to ask Product Creation Studio for additional updates. The prototype hardware needed additional features such as wireless charging as well as a risk-based approach to enhance performance, safety, and manufacturability. There was a key need for controls and monitoring of the drive and power level of the ultrasound transducer that delivered the therapy.
Critical for a device intended for unsupervised home use, Madorra identified the need for significant updates to the user interface behavior and Product Creation Studio implemented changes.
During the process, Product Creation Studio also identified the opportunity for a comprehensive suite of diagnostics and data logging capabilities. These were implemented collaboratively to support both internal testing and clinical needs.
There was a key need for controls and monitoring of the drive and power level of the ultrasound transducer that delivered the therapy. Product Creation Studio tuned the device hardware and developed a series of monitors and coherency checks to ensure reliable therapy delivery and safety.
Madorra chose to run the electrical engineering and firmware development under Product Creation Studio’s QMS, allowing their internal resources to focus on other aspects of the development. This collaboration enabled Madorra to cover more ground with a smaller full-time staff. A great synergy was established with the two teams routinely brainstorming together to solve some of the more challenging issues.
Product Creation Studio developed design outputs, test fixtures, calibration, and functional test procedures to support the selected contract manufacturer (CM) as they built the devices for clinical testing. These field trials demanded a significant quantity of devices to be produced under an ISO 13485 quality management system (QMS).
Project Results
The design collaboration between Madorra and Product Creation Studio resulted in devices for use in a take-home clinical study to test the early effectiveness of the ultrasound therapy. Product Creation Studio delivered a ruggedized, standards-compliant home ultrasound design. The updated circuitry, code, and improved user experience enabled Madorra to confidently approach their take-home field study milestone. Development under design controls ensured safety compliance and facilitated the field trials with unsupervised users.
Learn more about how founders Holly Rockweiler and Ryan Krone were inspired to create a non-hormonal solution for vaginal atrophy in this blog post from Stanford’s Byers Center for Biodesign program. You can also read up on Madorra’s most recent news, their FDA breakthrough device designation to treat moderate to severe vulvovaginal atrophy at home, in this Medical Design & Outsourcing article.


