Lumithera LT-300
Non-invasive, light therapy
LumiThera is at the forefront of laser and LED light treatment for sight threatening, acute and chronic ocular disease. Funded by the Life Science Discovery Fund (LSDF), LumiThera was chartered to develop a prototype medical device designed to treat dry, age-related macular degeneration (AMD) using non-invasive photobiomodulation (PBM). LumiThera partnered with Product Creation Studio (PCS) to bring their developmental stage LT-300 to the next level of funding and market readiness..
Services
Development Strategy, Product Design, Design Engineering, Innovation Management
Expertise
Mechanical Engineering, Firmware, User Experience, Electrical Engineering

Collaborative Science
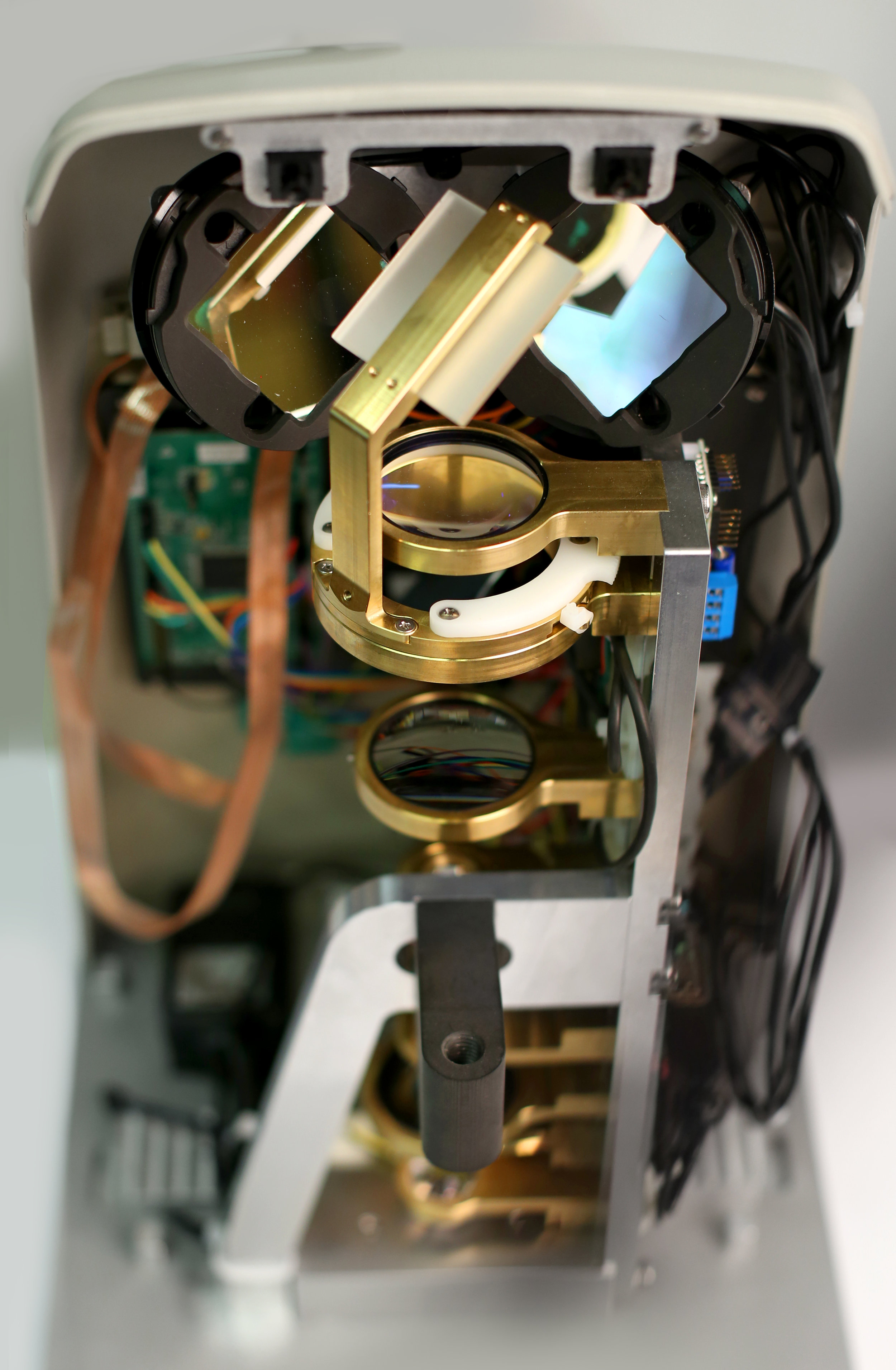
PCS worked with LumiThera’s scientists and near infrared light (NIR) experts to make the LT-300 a functional reality. The PCS team integrated LumiThera’s optics and light drivers, designing and engineering the platform, housing, patient interface, system electronics and firmware for proof-of-concept prototypes. Our teams then tested and optimized devices for early test and review by LumiThera’s board of medical experts.
Improving Lives
The collaboration between LumiThera and PCS achieved the next phase commercialization grant from LSDF and for our part PCS won the Most Innovative Company out of 2200 companies attending the MDM West Conference. Lumithera is now conducting human testing and in the Design for Manufacture (DFM) stage. PCS is proud to be part of the LumiThera team that will be the first to commercialize this impactful, non-invasive light treatment. This life-changing work will address vision loss, prevent blindness and improve the quality of life for people affected by macular degeneration.
In a late 2017 30-subject pilot study, LumiThera's LT-300 device posted topline, clinical interim data proving PBM improved the vision of dry AMD patients. You can read more about the study details and results in our blog post: Congrats to Client LumiThera on Posting Positive Clinical Interim Data!